Abstract
Introduction: Melphalan flufenamide (melflufen) is a first-in-class peptide-drug conjugate that targets aminopeptidases and rapidly releases alkylating agents into tumor cells. The single-arm, open-label, Phase 2 HORIZON study (NCT02963493), conducted in Europe and the USA, demonstrated in patients with heavily pre-treated relapsed/refractory multiple myeloma (RRMM), including those with triple-class-refractory and extramedullary disease, that melflufen in combination with dexamethasone showed clinically meaningful efficacy and a manageable safety profile (Richardson PG, et al. J Clin Oncol. 2021;39:757-767). Comparative trials between all regimens/agents are not feasible, and hence, real-world datasets may be valuable to allow the comparison of patient outcomes in single-arm clinical trials with similar patient populations in the real world. The objective of the REAL-world Myeloma (REALM) study was to compare clinical outcomes between patients in the COTA database and those of patients in the HORIZON study in order to compare routine care with melflufen.
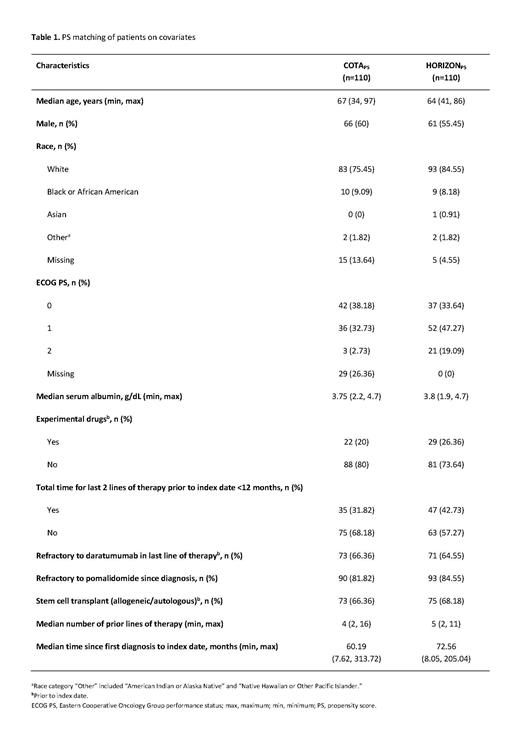
Methods: REALM is a retrospective, observational study using real-world data from 605 patients with RRMM collected in the COTA database, a USA-based real-world evidence database comprising longitudinal, Health Insurance Portability and Accountability Act (HIPAA)-compliant data on the diagnosis, clinical management, and outcomes of patients with cancer. Patients in REALM met the main HORIZON eligibility criteria (aged ≥18 years; ≥2 lines of prior therapy [including an immunomodulatory drug and a proteasome inhibitor]; refractory to pomalidomide and/or daratumumab on or after January 1, 2015; and received a line of therapy after meeting the inclusion criteria). Propensity score (PS) matching is a commonly used approach for comparing the effectiveness of 2 treatment options across different studies where individual patient data are available for both treatments. This abstract presents adjusted clinical outcome results based on 1:1 PS matching of patients in the COTA and HORIZON datasets for 12 covariates (COTA PS and HORIZON PS, Table 1). These covariates were considered important because they reflect patient characteristics that impact clinical outcomes and were validated in a feasibility study, and by independent experts. The primary endpoint of the HORIZON study was overall response rate. The primary endpoints of the REALM study were time to treatment discontinuation (TTD) and time to next treatment (TTNT), to mitigate against missing treatment response data in the COTA database identified in the feasibility study.
Results: Selected patient demographic and clinical characteristics were generally well matched (Table 1) between patients from COTA PS (n=110) and HORIZON PS (n=110), except for the Eastern Cooperative Oncology Group performance status, which was better in the COTA PS dataset than in the HORIZON PS dataset. Patients in both the COTA PS and the HORIZON PS datasets were predominantly male and White. Patients had a median age of 67 years in the COTA PS dataset and 64 years in the HORIZON PS dataset, with a median of 4 and 5 prior lines of therapy, respectively. The median time since first diagnosis to index data was 60.19 months in the COTA PS dataset and 72.56 months in the HORIZON PS dataset. The median TTNT (95% confidence interval [CI]) in the COTA PS dataset versus the HORIZON PS dataset was 4.6 (3.7-6.9) months and 5.9 (4.7-7.7) months, respectively (p=0.949). The median TTD (95% CI) in the COTA PS dataset versus the HORIZON PS dataset was 3.2 (2.6-3.9) months and 3.9 (3.0-4.6) months, respectively (p=0.642).
Conclusions: Pharmacoepidemiologic methods using individual patient data play an important role in evaluating the effectiveness of treatments in the absence of randomized controlled trial data. Patients with RRMM receiving melflufen in combination with dexamethasone noted a trend toward improved TTNT and TTD compared with corresponding real-world treatment approaches. This benefit may be clinically meaningful especially in the triple-class-refractory patient population, which remains an unmet need in RRMM.
Ailawadhi: Karyopharm: Consultancy; AbbVie: Consultancy; Genentech: Consultancy; Takeda: Consultancy; GSK: Consultancy, Research Funding; Xencor: Research Funding; Cellectar: Research Funding; Medimmune: Research Funding; Ascentage: Research Funding; Pharmacyclics: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; BeiGene, Ltd.: Consultancy; Sanofi: Consultancy; Oncopeptides: Consultancy. Wang: COTA, Inc.: Current Employment, Other: Equity ownership. Belli: COTA, Inc.: Current Employment, Other: Equity ownership. Jansson Blixt: Oncopeptides: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Cripps: Oncopeptides: Other: Independent consultant. Zavisic: Oncopeptides: Current Employment, Current holder of stock options in a privately-held company. Ramasamy: Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Conference registration, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Conference registration, Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Conference registration, Research Funding; Celgene (BMS): Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Conference registration, Research Funding; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Oncopeptides: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive biotech: Honoraria, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer oncology: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees.